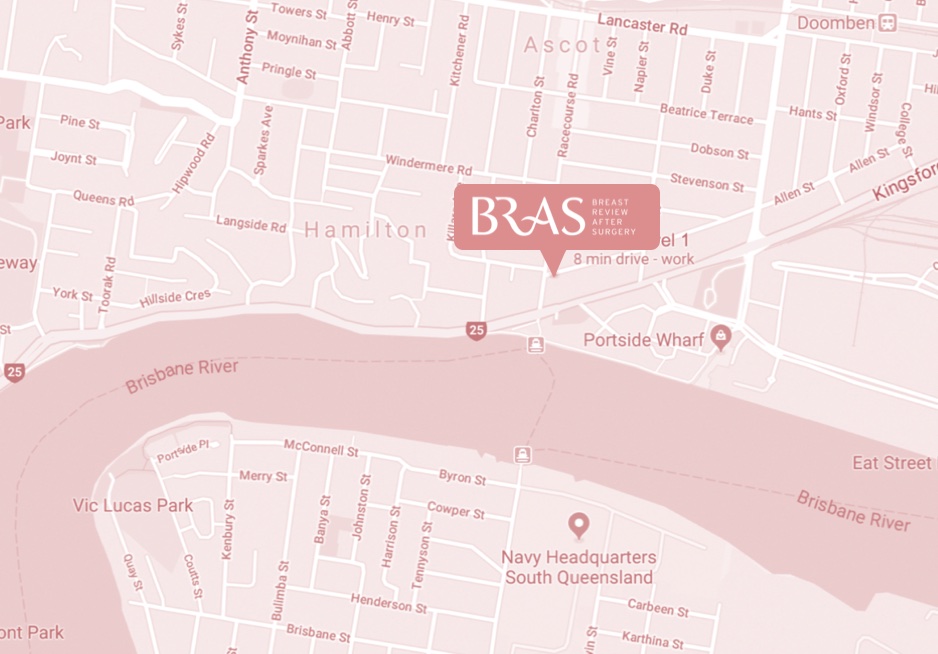
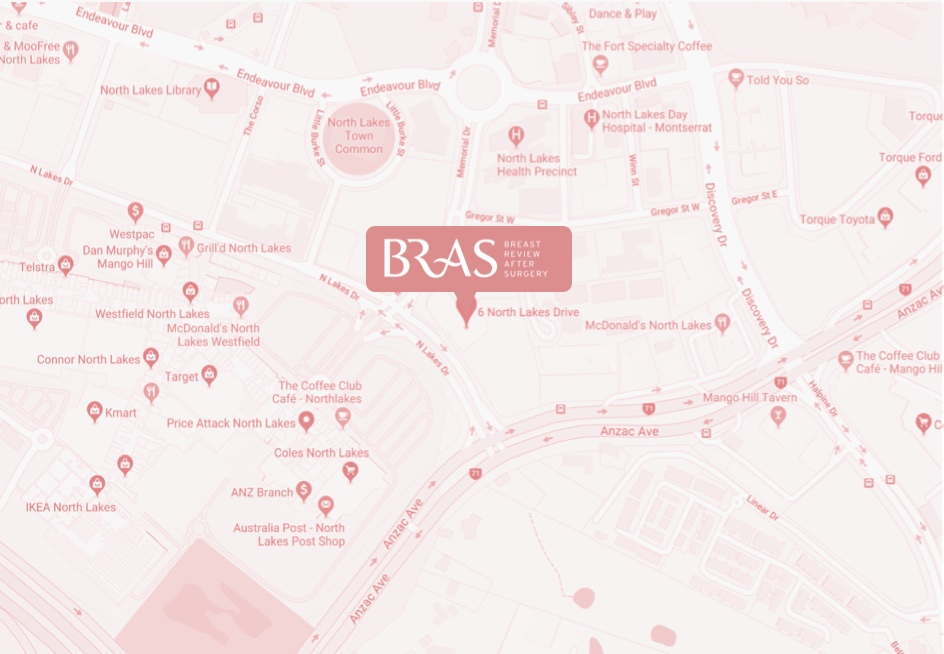
bulk-billed, obligation-free breast implant checks in Brisbane
Brisbane's First Breast Implant Review Clinic
about us
Designed to provide convenient, no-obligation breast implant checks in Brisbane, BRAS is ideal for patients who have post-operative concerns or breast augmentation complications, are dissatisfied with the results of their breast surgeon, or simply wish to have a general breast implant check-up and are not local to their operating surgeon.
BRAS clinic is NOT designed to provide breast cancer checks and is NOT suitable for any person who has undergone breast reconstruction surgery for diagnosed breast cancer or prophylactic reasons.

why choose BRAS
Why would I need it?
Patients will seek a breast implant check or breast implant ultrasound in Brisbane for a number of reasons such as:
– Post-operative breast implant complications & general post-operative concerns after breast augmentation
– Dissatisfaction with initial breast implant surgery for aesthetic reasons
– Seeking professional guidance regarding breast implant post-operative care and maintenance
Post-operative complications are the most common cause for a breast implant review, and there are a number of breast implant complications which can occur at varying degrees of severity, and assessment and treatment from a qualified health professional is important.
Types of Complications
Capsular Contracture
Capsular contracture is the most common reason for surgical removal and replacement of breast implants. This occurs when the tissue surrounding the breast implant tightens, resulting in discomfort/pain, firmness, and hardening of the breast. If severe, the capsule can constrict the implant and cause the implant to fold or rupture. Although the definitive cause of Capsular Contracture is unknown, currently there is strong evidence to indicate bacterial infection surrounding the breast implant is a major contributing factor.
Double Bubble
Double bubble in breast implants occurs when the implant shifts or migrates downwards into the breast crease causing a visible “bubble” at the inferior/lower aspect of the breast.
Rupture
Breast implant rupture can occur for numerous reasons, but often any rupture or leak remains contained and undetected in the tissue surrounding the breast implant. Occasionally this tissue becomes irritated resulting in pain, discomfort, swelling, changes in breast shape and/or size, or palpable lumps.
Bottoming Out
When a breast implant loses its internal support and your skin is not able to hold the implant properly in place it can begin to sink towards the lower part of the breast area. There is generally a larger distance between your nipple and inframammary fold, the bottom of your breast tissue may seem to bulge slightly downward, or your nipples may appear as if they are pointing more upwards than forwards.
Rippling
Breast implant rippling can cause noticeable ripples on the outside and underside of the breasts. This is more common in saline breast implants or women with naturally smaller breasts where there is less breast tissue to cover the implant. For women who have implants placed over the muscle rippling can be more common also.
Other Complications
There are also numerous other breast implant complications that patients may face including unexplained firmness or swelling or unexpected changes in the shape, size or feel of the breasts well after a patient has fully recovered post-operatively.
the team

Nurse Keren
Nurse Keren

FAQs
BRAS is designed for women who are generally at least 12 months post-breast augmentation and underwent initial surgery for aesthetic reasons. BRAS is NOT designed to provide breast cancer checks and is NOT suitable for any person who has undergone breast reconstruction surgery for diagnosed breast cancer or prophylactic reasons.
Patients will seek a breast implant check and breast implant ultrasound for a number of reasons including post-operative complications, general concerns, dissatisfaction with initial surgery, or to seek professional guidance regarding post-operative care and maintenance. Post-operative complications are the most common cause for a breast implant review, and there are a number of complications which can occur at varying degrees of severity, and assessment and treatment from a qualified health professional is important.
Located in Hamilton, Brisbane, BRAS is a nurse practitioner-led clinic run by Nurse Keren McKenna—Nurse Practitioner.
Your breast implant check in Brisbane with Nurse Keren will be billed directly to Medicare, with no out-of-pocket expenses. You will NOT require a referral to attend the clinic and if required, the clinic can organise a referral for ultrasound or to see a specialist Plastic surgeon for revisionary advice.
The appointment will take approximately 30 minutes.
blogs

Breast Implant Illness: Fact or Fiction?

Textured Implants vs. Smooth Implants
THE TGA & TEXTURED BREAST IMPLANTS
Post-Operative Care in Breast Surgery
All about BIA-ALCL (breast implant-associated large-cell lymphoma)
An Introduction to BRAS
 September 27, 2020
September 27, 2020
Breast Implant Illness: Fact or Fiction?
In recent years, the popularity of breast implants has increased significantly. This is in line with the growth of social media and the growing celebrity endorsement of cosmetic procedures. Alongside this growth in patient uptake has been an unsettling trend of women reporting a wide array of unpleasant and sometimes concerning symptoms that they believe to be associated with their silicone breast implants. This complex phenomenon has been referred to in the mainstream media and academic literature as “breast implant illness (BII)”. A quick look at the online landscape highlights the weight of the issue:
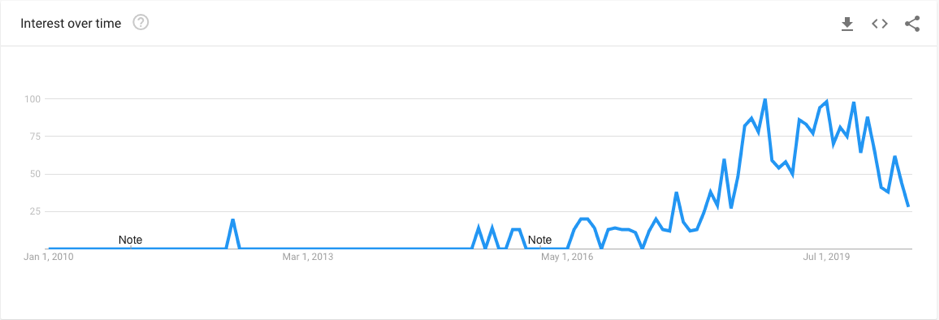
The above graph shows the google search trends for the term “Breast Implant Illness” over the past decade. As you can see, this topic really only gained traction in the past 4 years, reaching its peak in 2019. Likewise, this phenomenon has taken the social media world by storm, with thousands of women joining Facebook support groups dedicating to “Breast Implant Illness” healing.

The most popular of the groups is Breast Implant Illness and Healing by Nicole, with a huge membership of 125,000 women, averaging 300 posts per day. On top of these online communities is the countless news articles delving deep into the personal experiences of women with these self-reported symptoms. Among these articles, are stories of patients citing blurred vision, memory loss, body sores, heart palpitations, food intolerance, and more. One patient stated that she had spent over $30,000 on treatment for symptoms and medical advice to no avail, before implant removal was considered. This patient stated that after the removal of her implants, her symptoms disappeared. So what’s the deal? Do all patients experience breast implant illness? How do breast implants cause so many different symptoms? Is it even a real thing? There are certainly a lot of grey areas surrounding this topic, and this blog post aims to address just a few.
What is Breast Implant Illness?
Breast implant illness (BII) is a relatively new phenomenon, proliferating in recent years alongside the rapid growth in social media and the impact of online communications. There is no clear definition for the illness. Many sources loosely define it as a “term used by some women and doctors to refer to a wide range of symptoms developed following breast augmentation”. BII is not yet a recognised medical condition, and this can cause significant frustration in patients who feel their symptoms are a direct result of their breast implants.
The recency of this concept suggests that the shared experiences of women on social media fills a void for thousands of women across the globe that are lost and confused by their symptoms. The myriad of symptoms described can include—but are not limited to—the following:
- Joint pain
- Muscle pain
- Chronic fatigue
- Memory and concentration problems
- Breathing issues
- Sleeping difficulty
- Rashes and skin conditions
- Dry mouth and eyes
- Anxiety
- Depression
- Headaches
- Hairloss
- Digestive issues
- Rheumatoid arthritis
- Scleroderma
- Sjogren’s syndrome
With so many different symptoms (the list goes on), and so little uniformity in experiences, it can be difficult for many to understand BII. It is even harder to diagnose or treat a condition that does not technically exist yet. There is still a lot of contention in the public discussion and literature surrounding BII. So what is the science behind BII and how much do we actually know?
What is the science behind it?
There are many different views when it comes to research on BII; some corroborating, some conflicting, but all sharing the same sentiment that much more research is required to fully understand it. According to the FDA, the risk of complications or adverse outcomes due to breast complications is around 1%. Yet the FDA also reported statistically significant increases in fatigue, joint swelling, frequent muscle cramps, and fibromyalgia among augmentation patients. A study in the journal of Plastic & Reconstructive Surgery concluded that there was no association between breast implants and an increased risk of depression or neurological disease – two key self-reported symptoms associated with BII. However, newer studies did find an association between silicone breast implants and certain auto-immune diseases. This suggested the potential for increased risk of rheumatoid arthritis, Sjogren’s syndrome, scleroderma, and sarcoidosis. The FDA did not find their evidence to be sufficient however, and many experts state the findings were inconclusive. The National Cancer Institute (NCI) found a statistically significant increase in reported connective tissue disease among breast augmentation patients. This said, they also found self-reported symptoms to be flawed, for example patients reporting rheumatoid arthritis who actually had osteoarthritis. The findings suggested that there was an increase in symptoms for women with implants, but it is unclear whether there was an increase in specific diagnoses A study paper in the Journal of Annals of Internal Medicine reviewed the results of 32 studies regarding breast implants and health conditions and found no evidence to suggest silicone gel implants led to long-term health complications. It is clear from the existing research that there is little consistency in reported symptoms and each sample is vastly different, making it difficult to define a distinct syndrome. It is clear that at this stage, we do not have enough data to confirm or disconfirm BII, however the lack of a direct and scientific link does not indicate that the symptoms are not real.
According to Harvard Health Publishing , the results are inconclusive when it comes to defining breast implant illness. We are yet to figure out how breast implants could be the cause of such a constellation of symptoms. They state that some cases are likely due to undiagnosed auto-immune disease, and of the women who state an improvement in symptoms after explant, there is the potential for subconscious bias to believe the symptoms have gotten better. Dr. Pusic from Harvard Medical School states that there is a definite possibility however that some women have a susceptibility to problems related to breast implants.
What is the future?
As you can likely tell from this article, the whole concept of BII is messy, complicated, contradicting, and contentious. There are those who are suffering from debilitating symptoms they are certain are related to their implants, and there are those who are attributing these symptoms to placebo and believe self-diagnoses is unreliable. There are those who have been cured of their symptoms after removal, and those who are adamant that there is no scientific explanation for the existence of BII. What is the truth? The truth is we have a long way to go. More research, more recognition, and more interest in BII is required.
With so many women forming a community surrounding these concerning symptoms, it cannot be ignored. Whether these symptoms are truly a result of their breast implants is yet to be understood, but the anecdotal evidence is too strong to be disregarded.
Do you have breast implants? What’s your take on BII? Have you experienced any concerning symptoms since your surgery? Contact the BRAS clinic today for your routine breast check and ultrasound, entirely bulk-billed.
 January 15, 2020
January 15, 2020
Textured Implants vs. Smooth Implants
Whether you are a woman considering breast augmentation or a woman with breast implants that will consider re-operation in the future, the choice of implant shell is an important factor that you will take into account during the planning of your surgery. Implant shells come in a number of different varieties, and can be largely categorised into two main types: smooth and textured. Over the past few years there have been significant changes in the industry, with a distinct shift in the preference of plastic surgeons regarding their preferred implants. This blog post aims to outline the various pros and cons of both smooth and textured implants to equip women with the right knowledge to make the best decision for them.
Breast Implant History
The first silicone gel breast implant was manufactured in 1962. A decade later the second generation of gel implants was introduced to the market featuring a silicone shell, gel, and polyurethane textured surface which produced a superior aesthetic outcome and reduced the risk of capsular contracture. However, due to safety concerns relating to the breakdown product of polyurethane these implants were taken off the market and a new textured surface was introduced. This textured surface intended to serve similar benefits to the polyurethane implant by reducing capsular contracture rates. There was a subsequent period where saline implants were widely used, shifting the implant shell preference to a smooth silicone shell as this reduced rates of rippling and deflation. Silicone implants became the most common implant type used by surgeons due to further advances in the technology and the limitations of saline implants such as visible deflation following rupture. Textured surfaces remained the preferred option due to the stability in the pocket and aesthetic consistency however over the past decade new findings have emerged that indicate a rare link between some textured implant varieties and breast implant-associated anaplastic large cell lymphoma. We have written a blog post about this rare condition which you can read here.
While a number of textured implant varieties have been banned or suspended under investigation due to this rare link, there are many plastic surgeons who still choose to use textured implants and many patients that still choose this shell. This said, there are also many plastic surgeons that have sought out alternative options to ensure they uphold the highest safety standards for their patients. You may wonder why patients and surgeons would continue to use textured implants when there is a possible link to this rare lymphoma. It’s important to consider the various strengths and limitations of each implant shell variety to better understand the rationale for each.
Textured Implants Pros and Cons
Strengths
- Reduced risk of capsular contracture. The irregular surface texture of the textured breast implant serves to disrupt the planar arrangement of fibroblasts which can prevent contraction.
- Predictability of placement. The textured implant has a more predictable result with greater adherence to the breast capsule.
- The textured surface can present resistance to movement through friction which has benefits including low rates of bottoming out and malposition.
Limitations
- The use of textured implants requires exact pocket dissection to ensure optimal results as there is little movement within the pocket and aesthetic results are less forgiving when the pocket is not correctly positioned.
- There is a higher risk of late seromas and double capsules.
- There is a link between textured implants and BIA-ALCL
Smooth Implants Pros and Cons
Strengths
- Can produce a more natural shape and appearance. The smooth outer-surface of the implant can sit more naturally in the pocket and create a more natural look and feel.
- More forgiving aesthetically when dissecting the implant pocket. The implant will move naturally within the pocket to create a more natural appearance.
- Latest generation implants have less wrinkling as the gel is continuous with the outer shell as opposed to separate.
Limitations
- Higher capsular contracture rates
- Greater mobility within the pocket. The implant naturally settles to the bottom of the pocket which may stretch the lower pole over time or cause malposition
It is clear from the various strengths and limitations of each shell that the answer of which implant is best is not straightforward, as patients may have different priorities. The question is, is there a happy medium? Is there an implant shell that can synergise the two types? The newest generation of implants suggests that SilkSurface nano-texturing is the way forward.
Motiva Implants
Dr. Phil Richardson from Brisbane Plastic & Cosmetic Surgery explains that his implant of choice is Motiva implants. Technically these ‘nano-textured’ implants are classified as a smooth implant, thus eliminating the potential risk of BIA-ALCL, which is a huge factor against the use of textured implants. However in to traditional smooth implants, Motiva are characterised by a rigid topography with thousands of contact points per cm to encourage the optimal cellular response and reduce the risk of capsular contracture. The capsular contracture rate for Motiva is less than 1%. There are a number of additional benefits of this new technology including their advanced nanotechnology reducing bacteria and biofilm formation as well as inflammation, and their unique surface reaping the benefits of stability in the pocket that many textured implant varieties historically held against smooth options. The overall complication rate for Motiva implants is 0.36%. This new generation of breast implants seemingly bridges the gap between smooth and textured implants to leverage the strengths of both shells.
Should I choose textured or smooth implants?
If you are a woman considering breast implants, you should consider the various pros and cons of all options at your disposal. You should have a thorough discussion with your plastic surgeon, as depending on your anatomy and medical history you may be recommended a certain shell for unique reasons. It’s important to stay up to date with the latest research in breast implant technology to better understand which implant is pioneering safety and aesthetic outcomes, and it’s equally important to consider what your priorities are. For some patients, the risk of BIA-ALCL is not a factor that will deter them from choosing a textured implant that has lower risk of capsular contracture and movement in the capsule, while for others this risk is substantial and a clear rationale for the use of a smooth implant. Once you have an understanding of what the facts and your own needs, you can then take the steps towards surgery.
If you have any questions about implant shells or would like to learn more, contact us at BRAS clinic today.
THE TGA & TEXTURED BREAST IMPLANTS
Were textured breast implants banned?
On the 2nd of August, 2019, the TGA posted an announcement that implant manufacturer Allergan was issuing a voluntary recall of their macro-textured breast implants and tissue expanders (this recall applied to current shelf-stock, and not patients who currently have these breast implants). Since this announcement, the public has been awaiting a verdict from the TGA regarding the action they choose to take on other varieties of textured breast implants. On the 26th September this update was posted, and created significant media attention and patient concern. The update stated that the TGA has decided to take regulatory action in relation to all breast implants and tissue expanders sold in Australia. This includes the suspension (not ban) and recall of a few select implants, namely the Nagor, Emagin, Cristaline, Sublime, and 4Two line varieties. The suspension states that these devices will no longer be supplied, imported, or exported over the 6 month suspension period, and that all un-implanted products will be subject to recall (ie. those products on hospital shelves). The TGA also states that this suspension can be revoked if concerns about the devices are addressed by manufacturers to the TGA’s satisfaction.
In addition to the announcement of this suspension, the TGA includes a table with an extensive list of implant varieties that were subject to a set of conditions. These conditions include more involved patient and practitioner education, and more comprehensive paperwork. There was significant confusion regarding this section of the article, as many patients believed this extensive list of implants are subject to recall. It is important to note that the implants in this secondary table are simply under new guidelines with regard to reporting, instructions for use, and patient information. These implants are not being considered for suspension, nor recall.
Why were textured implants suspended?
The rationale behind the suspension, recall, and imposition of additional conditions lies in the reported link between textured breast implants and anaplastic large cell lymphoma (ALCL). Breast implant associated ALCL (BIA-ALCL) is a rare disease of the T-cells, which is said to be caused by a reaction to bacterial growth. Researchers propose that due to the high surface area of some textured implant varieties, they can create a host for bacteria and may increase the likelihood of ALCL developing for some patients. The current estimated risk rate varies depending on the type of implant, but can be between 1 in 4,000 for more highly textured (polyurethane) implants, to 1 in 86,000 for lower surface area implants. The risk remains extremely low, and can also be caused by genetic predisposition. To date, there have been no cited cases of BIA-ALCL in women with smooth implants. The link between breast implants and ALCL should not be of significant concern to patients with breast implants. According to the Australian Society of Plastic Surgeons, conservatively there are 35 million women in the world with textured implants. Of this 35 million, there are just over 500 confirmed cases of BIA-ALCL. This suggests the disease affects an extremely small percentage of the total breast implant population, which is predicted to decrease over time as existing patients are educated regarding the signs, symptoms, and proper follow-up, and future patients are implanted with smooth surface varieties.
What are the symptoms of ALCL?
For patients with textured implants concerned about BIA-ALCL, there are a number of signs and symptoms to be aware of. Firstly, any changes in the appearance or feel of the breasts should be flagged with your operating surgeon, or a qualified review clinic such as The BRAS Clinic. A key symptom to look out for is a large amount of fluid in the breast capsule developing, with a dramatic change in the volume or size of the breast that is not related to other pain or trauma. If you experience these symptoms, it is recommended that you have a routine ultrasound as well as a fine-needle aspiration to test the fluid in the implant for ALCL. When detected at this early stage, ALCL is very treatable as it is contained to the capsule, and can be treated through removal of the implant and capsule.
What should I do if I have textured implants?
The BRAS Clinic recommends for all patients with textured implants to not be concerned or alarmed by the TGA’s announcement. The governing body has made clear that there is no recommendation to have these implants removed or replace, as the risk of surgery and general anaesthesia is far greater than that of BIA-ALCL. Rather, we recommend a physical check-up at the BRAS Clinic where you will receive a referral for a routine ultrasound to ensure there is no excessive fluid around the implant, and no signs of rupture or other complications. You can make an appointment with the BRAS Clinic by contacting us today.
What breast implants should I get if I am considering breast augmentation?
If you are a patient considering breast augmentation, you should be thinking about mitigating future risk by choosing a smooth surface implant. The BRAS Clinic strongly recommends Motiva implants, as they are the most advanced, and safest implant technology on the market. Their silk-surface nano-technology means that each contact point of the Motiva implant surface is pre-determined and smaller than a single cell, preventing the inflammatory process progressing and reducing the risk of ALCL.
Post-Operative Care in Breast Surgery
An integral aspect of the cosmetic breast surgery journey which is often overlooked by patients during their initial research phase is post-operative care. Patients focus heavily on aesthetic outcomes, expertise, and other important factors, often forgetting that post-operative care is a pivotal aspect of a positive experience. Post-operative care is important for a number of reasons:
Firstly, complications can always occur. Regardless of how experienced your surgeon may be, or how many measures you take to prevent complications, they can still occur. Some complications such as capsular contracture, are oftentimes out of the control of both surgeon and patient, and can happen at any point following surgery. Having the proper support and services in place to address such issues when they arise is undeniably important.
Secondly, many complications may go undetected, such as rupture. While this complication may not be debilitating or a health risk, it is important to identify if rupture is present through ultrasound, to ensure the longevity of your results and flag the possible need for re-operation.
Thirdly, there are some more rare and serious consequences that have evidenced themselves in recent years with regard to textured breast implants. We have discussed the issue of breast implant-associated ALCL in a previous blog post, and stress the importance of early detection to ensure your safety. This is a particularly serious reason that post-operative care should not be taken lightly. While the risk of BIA-ALCL is as low as 1 in 40,000, it is so important to remain vigilant and aware of any symptoms, and even more importantly, stay on top of your routine check-ups.
Some questions you should ask yourself when choosing a surgeon, are:
- Does this surgeon offer long-term care?
- Do they recommend routine ultrasounds post-operatively?
- What measures do they have in place to ensure my long-term safety?
Ask your surgeon whether they offer a complimentary lifetime follow-up plan. This is a measure that some surgeons such as Dr. Philip Richardson from Brisbane Plastic & Cosmetic Surgery are taking in conjunction with BRAS Clinic to ensure a gold standard of care and a prioritising of patient safety. A lifetime follow-up plan should include two-yearly or yearly check-ups which involve providing referrals for an ultrasound of the breasts, a physical examination at the clinic, and a review summary including relevant recommendations.
These review appointments will provide you with peace of mind knowing that your breast implants are intact with no evidence of rupture, capsular contracture, fluid accumulation, or any other complications that can occur during the post-operative period. The alternative is to have undetected complications that may cause more serious consequences in the future.
It’s also important outside of your regular review appointments to be aware of possible symptoms to look out for. Dr. Richardson outlines a number of symptoms that should be flagged with your operating surgeon or an onsite nurse in the event that they do occur:
- Unusual and significant pain after the first year
- New onset/sudden swelling of one or both breasts
- Redness in one or both breasts / feeling feverish
- Issues with the incision site/scar healing
- Hardening of one or both breasts, causing discomfort of visible distortion
- Visible and notable changes in the shape or appearance of breasts
If you experience any of the above, or any other symptoms that are of concern to you, we encourage you to make an appointment at BRAS clinic to have a routine check-up. BRAS Clinic provides post-operative review services to women with breast implants at no out-of-pocket expense, and with no obligations.
The premise of the clinic is to help women who have had cosmetic breast surgery stay on top of their breast health, and ensure their safety in the long term by providing accessible services. If you feel you are due for your breast implant check, contact us today.
All about BIA-ALCL (breast implant-associated large-cell lymphoma)
The topic of ALCL and breast implants has become inherently linked in recent years, given the discovery of a rare type of lymphoma associated with textured-surface breast implants. Over the past decade, there have been a number of reported cases of ALCL in women with textured breast implants. There are important facts and statistics to consider when reviewing the topic, and we’ll try to get through the most important of them in this blog post.
What is ALCL?
The Leukaemia Foundation provides us with an apt definition of Anaplastic Large Cell Lymphoma (ALCL), describing it as a rare type of non-Hodgkin lymphoma made up of either malignant T-cells (the cells in the immune system) or “Null-lymphocytes”. It occurs in two forms: one which is systemic and aggressive, affecting all organs in the body, or one which is cutaneous, slow-growing and confined to the skin. It is a rare disease of which the cause is unknown. The Macmillan Cancer Foundation present a definition in lay terms, stating that ALCL develops when white blood cells called T-cells become abnormal, generally building up in. The lymph nodes and potentially affecting other areas of the body. This article also states that the condition is more common in children and young adults. The facts are slightly different however when it comes to breast implant-associated ALCL (BIA-ALCL).
What is BIA-ALCL?
The TGA defines BIA-ALCL as a rare cancer of the immune system which is not breast cancer (which forms in the cells of the breast) but rather a cancer that grows in the fluid and scar tissue which has formed around a breast implant. There is an important distinction between BIA-ALCL and breast cancer. It is extremely important to note that this rare disease has historically only been associated with textured-surface implants. There are no cited cases of BIA-ALCL in patients with smooth implants.
The Australian Society of Plastic Surgeons states that it takes on average 7-10 years before the disease develops in a woman who has had breast implants. They state that early stage disease is curable with surgery alone, while if the disease has spread through the capsule to local lymph glands, the prognosis is less favourable. As such, it is important to recognise the symptoms.
Why have this disease been associated with breast implants?
There have been a number of articles considering the possible role of textured breast implants in causing BIA-ALCL. There are several unifying factors that have been posited to cause the condition.
- Textured breast implants create high surface area textures that increase the risk of bacterial contamination
- Bacterial contamination at the time of surgery can reach a threshold that causes inflammation
- The patient has a genetic predisposition to ALCL
ASPS considers that bacteria has been identified as a causation factor of ALCL, and textured implants increase the risk of bacterial contamination due to their high surface area texture.
What is the likelihood of getting BIA-ALCL?
The current literature provides a variety of statistics regarding BIA-ALCL risk, dependant on the type of implant. In Australia, the risk of BIA-ALCL in patients with implants that have a high surface area texture is 1 in 4000 to 1 in 7000. These implants include Biocell, Allergan, Polyurethane, and Silimed. In contrast, the risk of patients that have implants with a lower surface area texture is much less, 1 in 60,000. These implants include Siltex and Mentor. There is currently no cited risk of BIA-ALCL in patients that have smooth surface implants, and it is proposed that this is due to the reduced risk of bacterial contamination. An example of a smooth implant is the Motiva implant. En masse, the risk of BIA-ALCL remains extremely low, particularly for implants with a lower surface area.
What are the symptoms of BIA-ALCL?
In order to ensure early detection and successful treatment, patients should remain vigilant in their monitoring of changes in the breast. The most common symptom is significant and late onset swelling in one or both breasts caused by fluid build-up. This can also present itself as a lump in either the breast or armpit. A degree of swelling post-surgery is certainly normal, however all concerns should be raised with a qualified healthcare practitioner, particularly if you see a dramatic change in the volume or size of the breast.
Once swelling has been assessed by your surgeon, they may refer you for an ultrasound. BIA-ALCL cannot be detected through mammogram, and an ultrasound is necessary to diagnose the swelling. In the instance of fluid being the cause of the swelling, a fine needle aspiration will be performed to take a sample for analysis. The majority of fluid build-ups are not associated with BIA-ALCL, however it is always best to be vigilant and address these concerns.
What are the treatment options for ALCL?
The treatment plan for BIA-ALCL is subject to the stage of the disease. According to the American Academy of Family Physicians, the majority of the information about treatment describes removal of the implant as well as the capsule surrounding the implant, and for some patients treatment with chemotherapy and radiation is required. Treatment plans are determined by the practitioner, and for most cases that are detected early when patients experience onset swelling or unusual lumps when they appear, removal of the implant and the capsule is sufficient to treat the disease, as it has not yet spread beyond the capsule.
What if I have textured breast implants?
The TGA suggests that BIA-ALCL is extremely rare, and as such, it is not recommended to remove your implants if you are a healthy person with no symptoms. The risk of this disease is as rare as 1 in 40,000 compared to the 1 in 8 who are at risk of breast cancer (regardless of whether they have implants). As such, the most important thing to consider if you do have any type of breast implant is to remain vigilant with your breast checks and appointments. Dr. Philip Richardson from Brisbane Plastic & Cosmetic Surgery in a recent article suggests that patients should be contacted by their operating surgeon every two years to receive an ultrasound and have a review appointment to ensure that patients are staying on top of their health and possible symptoms. Yet plastic surgeon consultation can be hefty, and patients may be reluctant to fork out any money when they believe there are no issues. BRAS Clinic aims to provide review services and ultrasound referrals to patients with no out-of-pocket costs, and no obligations. The goal is to ensure optimal safety and long-term maintenance for all patients. If you think you may be due for a breast implant review, please contact us.
What if I am considering getting breast implants?
For those considering breast implants, we encourage you to continue your research and to preferably choose a smooth surface implant to mitigate the potential risk associated with textured surfaces. It is important to ensure you are always choosing a fully qualified plastic surgeon who follows the 14-point plan to minimise bacterial contamination. There are a number of considerations that need to be made when choosing a surgeon including their safety measures, the type of implant they use, the hospitals they operate in, and the number of years’ experience they possess. Always do your research, and if you would like more information on how to choose a plastic surgeon, do not hesitate to contact us.
What next?
Research surrounding the causes and treatment of BIA-ALCL continues to this day, and measures to ban textured implants are currently being placed under an industry spotlight. To stay up to date with the latest news and policies surrounding BIA-ALCL, make sure to follow us on Instagram @brasbrisbane
An Introduction to BRAS
BRAS is a nurse-practitioner led clinic that offers professional review services for women who have previously undergone breast surgery. Founded by Dr. Philip Richardson from Brisbane Plastic & Cosmetic Surgery and run by Nurse Keren McKenna, BRAS is an all-female clinic designed to provide women across South-East Queensland with a safe and supportive environment to discuss their post-surgery concerns or questions.
Dr. Philip Richardson is a Plastic, Reconstructive & Cosmetic Surgeon in Queensland. Over his 16 years as a practising plastic surgeon, he has performed hundreds of revisional surgeries on patients from far and wide who are unhappy with their initial surgery, or are experiencing post-operative complications. Not all patients who book a consultation are seeking re-operation and more often than not, are just seeking advice and recommendations for alternative treatment plans. Over the years, as the number of breast surgery patients increases in Queensland, there is a growing trend of patients seeking professional advice and guidance in regards to their implants. Consultations with plastic surgeons can be expensive, and sometimes daunting for patients who are unhappy with their results, are not ready for re-operation, or are emotionally impacted by a negative experience.
BRAS is designed to support these women through a no-obligation review service that provides tailored advice and treatment plans at a fraction of the cost. The idea is to deliver a nurturing and supportive all-female environment for these women. Nurse Keren McKenna has worked in the plastics industry for 17 years, working alongside numerous plastic surgeons across Queensland as both a surgical assistant and practice nurse. Her knowledge of cosmetic breast procedures is extensive, and she has provided post-operative care to countless women across the state. Under the guidance of Dr. Richardson’s recommendations and post-operative care protocol, she will provide patients with a comprehensive review and discussion to determine their best treatment plan. The cost of the review is $70, with a $49.80 rebate from Medicare. As such, the out of pocket expense is just $20.20.
If you are a patient seeking professional guidance with regard to your breasts after cosmetic surgery, BRAS clinic is available to provide you with support and a plan of action.
Fill out your form on the “book now” tab of our website today to organise a review.
contact
MON-THU
8.30AM - 5PM
FRIDAY
8.30AM - 4PM
CLOSED ON WEEKENDS